Windows,Android apps for Engineers/ Students / Educational / Sports / Health personnel etc.
CRITICAL CONSTANTS HELP
|
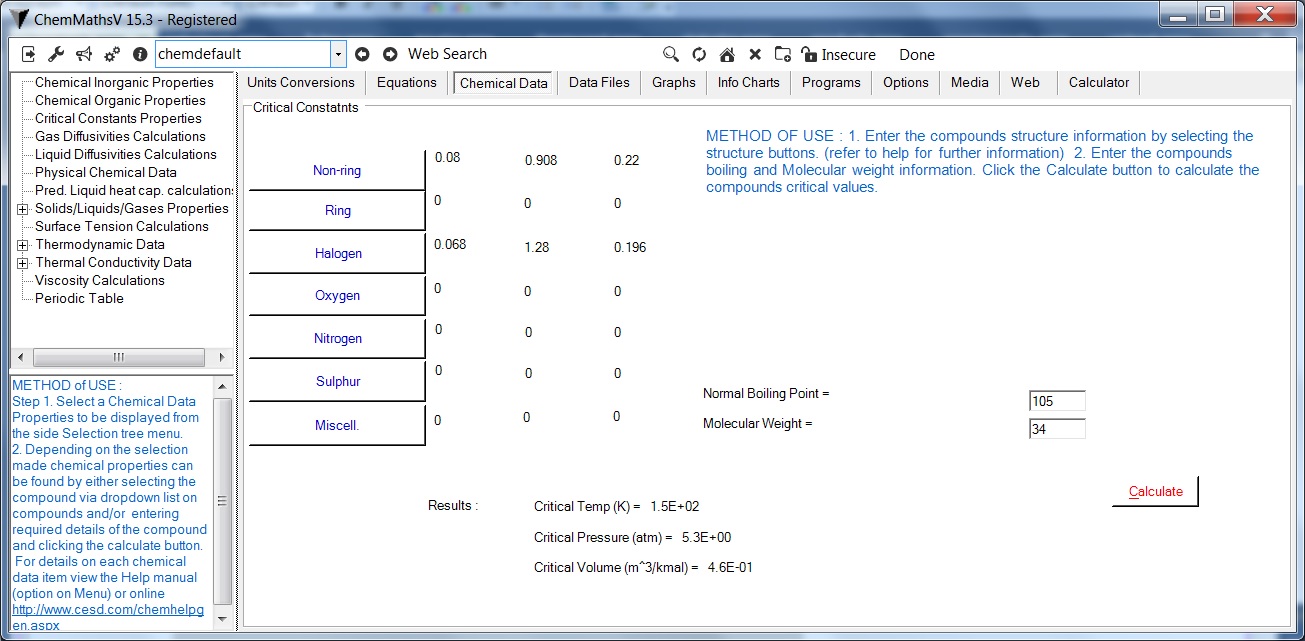
Prediction of the critical constants of compounds.Using Lydersen method
The Equations used are :
Tc = Tb/[0.567 + ΔT- (ΔT)2]
Pc = M/(0.34 + ΔP)2
Vc = 0.04 + ΔVlaycb">Tc = critical temperature, k.
Pc = critical pressure, atm
Vc = molar volume at the critical conditions, m^3/kmol.
Tb = normal boiling point, k.
ΔT, ΔP, ΔV = critical temperature, pressure and molar volume increments. All calculated using Lydersen, 1955 data points.
|
|
Method of use :
Determine chemical groups of the compound structure.
Select contribution value of each group via relevant group structure buttons.
For each group enter the number of times it appears in the compound .i.e. compound has 2 methyl groups enter 2 in associated text box. Click okay button when completed.
Enter molecular weight , normal boiling point, Click Calculate, predicted critical temperature, pressure, volume of the compound calculated.
Example diphenylmethane :
Group Number ΔT ΔP ΔV
C 10 0.11 1.54 0.37
= C –(ring) 2 0.022 0.308 0.072
--CH2-- 1 0.02 0.227 0.055
Totals: 0.152 2.075 0.497
Hence : Predicted Critical values via calculations are :
Tc = 772 k
Pc = 28.8 atm
Vc = 0.537 m3/Kmol
×
![]()